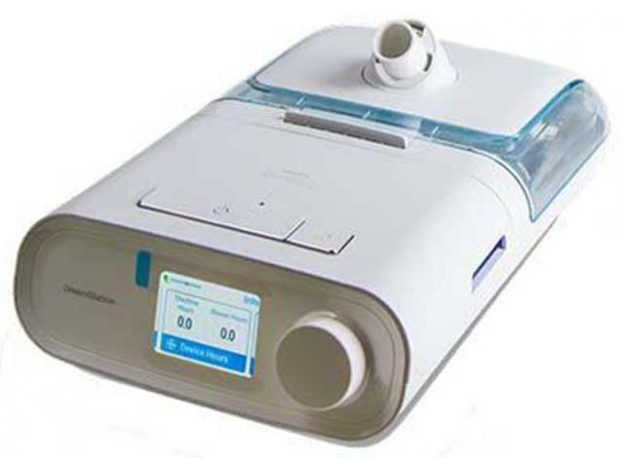
CPAP machines, (standing for Continuous Positive Airway Pressure) have been around for years and approximately 2.5% of our population uses them for a variety of reasons, with the most common being sleep apnea and snoring. These small electrically powered air pumps, deliver just the right pressure of filtered and humidified air into a wearer’s nose via a mask and hose, to keep their airway clear when sleeping. It’s been estimated that up to 50% of adults have some type of sleep apnea (pauses in breathing when sleeping). It’s a serious problem, because sleep apnea sufferers don’t get much value or rest from their sleep, leading to drowsiness during the day and a marked increase in the risk of a stroke. If a patient is diagnosed with a serious case of apnea, (a process which requires overnight testing in a sleep lab), and they don’t follow through with their physician’s instructions on getting and using a CPAP machine, this may result in the suspension of their driver’s license.
A recently released recall notice of one of the more popular brands of these machines should have users checking their brand and serial number to see if the devices should continue to be used or packed away. Philips Respironics announced a recall for Continuous and Non-Continuous Ventilators (certain CPAP, BiLevel PAP and Ventilator Devices) due to two issues related to the polyester-based polyurethane (PE-PUR) sound abatement foam used in these devices. For information on the Medical Device Recall, a complete list of impacted products, and potential health risks, visit philips.com/src-update.
CPAP users fall into 2 categories; those that must use them to sustain life and those that use them as part of apnea treatment. For patients using life-sustaining mechanical ventilator devices, the statement on the manufacturer’s website states, “Do not stop or alter your prescribed therapy until you have talked to your physician. Philips recognizes that alternate ventilator options for therapy may not exist or may be severely limited for patients who require a ventilator for life-sustaining therapy, or in cases where therapy disruption is unacceptable. In these situations, and at the discretion of the treating clinical team, the benefit of continued usage of these ventilator devices may outweigh the risks. If your physician determines that you must continue using this device, use an inline bacterial filter. Consult your Instructions for Use for guidance on installation.”
For those using these devices as treatment only the advice is to “discontinue use of your device and work with your physician or medical equipment provider to determine the most appropriate options for continued treatment.”
Phillips Respironics is in the process of notifying owners by mail, but via their website (mentioned above) you can register your contact information for quicker and more direct information.